How to Find Valence Electrons: A Practical Guide for Students in 2025
Understanding Valence Electrons
Valence electrons are important as they play a crucial role in chemical bonding and determine the chemical properties of elements. These **electrons** reside in the outermost electron shell (the **valence shell**) of an atom. Understanding how to find valence electrons is essential for students studying chemistry. This knowledge not only aids in predicting an atom’s **bonding** behavior but also enhances comprehension of periodic trends and group configurations on the **periodic table**. By mastering the skills of counting and determining valence electrons, students can develop a solid foundation in chemistry that supports their studies in molecular bonding, stability, and chemical interactions.
What are Valence Electrons?
Valence electrons are defined as the electrons in the outer shell of an atom that can be involved in forming bonds with other atoms. The number of **valence electrons** influences an atom’s **bonding characteristics** and chemical reactivity. For elements found in the same group of the **periodic table**, the number of valence electrons typically remains consistent; for example, alkali metals have one valence electron, while noble gases typically have eight, contributing to their chemical stability. Identifying these electrons helps predict the **oxidation states** of elements and their reactions in chemical processes – knowledge crucial for anyone studying **organic chemistry** or engaging in **chemical reactions**.
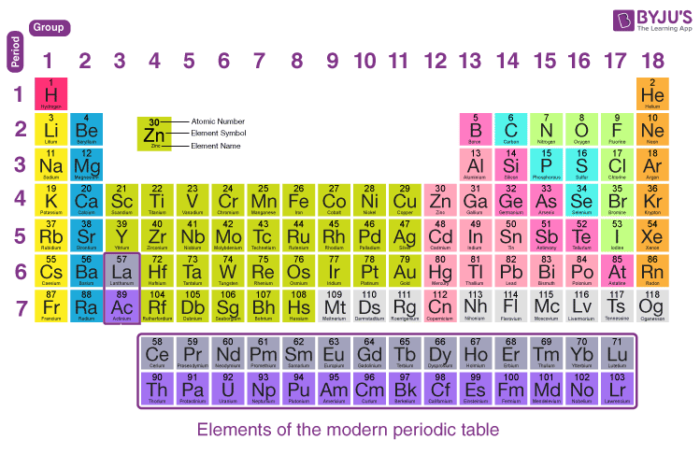
The Role of the Periodic Table in Finding Valence Electrons
The **periodic table** serves as a vital tool for determining valence electrons. Each element’s position within the table directly corresponds to its electron configurations and the number of valence electrons. For instance, elements in Group 1 have one valence electron, while those in Group 2 have two. Groups 13-18 align with the respective numbers of valence electrons ranging from three to eight. Understanding this systematic arrangement helps students quickly identify the characteristics of different **elements**, their potential **bonding**, and how they behave chemically within larger **molecular structures**.
How to Determine Valence Electrons Using Electron Configuration
To determine the number of valence electrons, it’s essential first to understand **electron configuration**. This refers to the distribution of electrons among the atomic orbitals of an atom. By following the **Aufbau principle**, electrons fill orbitals in order from lowest to highest energy levels. The configuration reveals the electrons in the **valence shell**. For example, the electron configuration of carbon (C) is 1s² 2s² 2p², indicating that it has four valence electrons located in the second shell. This method can be generalized for all elements, allowing a systematic approach to identifying **valence electrons** critical for molecular interactions and chemical stability.
Counting Valence Electrons
Counting valence electrons is fundamental in predicting how an atom will bond with others. Understanding the method behind counting allows students to visualize and predict outcomes of **chemical reactions** accurately. Here, we’ll explore several techniques for counting these vital electrons effectively.
Using Lewis Dot Structures
One of the most effective methods for visualizing **valence electrons** is through **Lewis dot structures**. These diagrams represent the valence electrons of an atom as dots surrounding the element’s symbol. For instance, carbon, with four valence electrons, is depicted as “C:.” To draw a Lewis structure, students must first determine the valence electrons and then distribute them around the element symbol, pairing them when necessary to represent **bonding** and fulfill the stable configurations in **molecular structures**. Practicing this skill reinforces the understanding of how atoms combine and the resulting **chemical behavior**.
Application of the Octet Rule
Applying the **octet rule** can simplify counting valence electrons. This rule states that atoms tend to gain, lose, or share electrons to attain a full set of eight electrons in their valence shell, resulting in more stable electron arrangements. Elements on the left side of the periodic table, such as sodium, typically tend to lose electrons, while those on the right, like chlorine, commonly gain electrons. It is crucial for students to practice identifying which structures adhere to the octet rule throughout chemical bonding to understand **stability** and the **properties** of resulting compounds accurately.
Examining Group Trends on the Periodic Table
Group trends in the **periodic table** provide a quick reference for determining the **number of valence electrons**. For example, as you move down a group, despite the increasing atomic size, the number of valence electrons remains constant. This consistency assists in recognizing patterns in **bonding** characteristics and **chemical stability** across different elements. Familiarity with these group characteristics helps students predict behaviors in complex reactions more effectively.
Valence Electrons and Chemical Bonding
The relationship between **valence electrons** and **bonding** is integral to understanding and predicting the behavior of chemical compounds. The way atoms interact with their electrons significantly influences their reactivity and bonding patterns. By grasping these concepts, students can develop a stronger comprehension of **chemical reactions** and **stability**.
Covalent and Ionic Bonds
Understanding the types of bonds formed based on **valence electrons** is critical in chemistry. Atoms that share their valence electrons form **covalent bonds**, while those that transfer electrons from one atom to another create **ionic bonds**. For instance, in the formation of sodium chloride (NaCl), sodium donates one electron to chloride, resulting in a stable ionic compound due to electrostatic attraction. Recognizing how electrons participate in different types of bonding facilitates a better understanding of compound formation and **chemical equations**.
Valence Electron Interactions in Molecular Geometry
The interaction of **valence electrons** not only influences bonding but also impacts **molecular geometry**. The spatial arrangement of atoms in a molecule arises from the repulsion between the **electron domains** around the central atom. Knowing the electron distribution allows for accurate predictions regarding the shape of molecules—vital for understanding reactions and properties of substances in chemistry. Understanding concepts such as **VSEPR theory** can help students gain insights into how geometry affects molecular interactions.
Influence of Electronegativity and Valence Electrons
**Electronegativity** influences how valence electrons are shared in a chemical bond, affecting the molecular structures formed. Differences in electronegativity between bonding atoms determine polarity, which, in turn, influences the compound’s stability and reactivity. For example, when examining the hydrogen fluoride (HF) bond, fluorine’s higher electronegativity causes it to attract the shared electrons more strongly than hydrogen, leading to a polar bond. Recognizing these differences equips students to predict and understand chemical properties and reaction mechanisms better.
FAQs on Valence Electrons
1. How can I find the number of valence electrons quickly?
Quickly identifying the number of **valence electrons** can be done using the periodic table. Each column corresponds to a specific number of valence electrons. By knowing the group number of an element, such as Group 1 or 2 for valence counts, students can determine the number of electrons efficiently. For transition metals, the process is nuanced, requiring understanding of their electron configuration.
2. What is the significance of valence electrons in chemical bonding?
Valence electrons are pivotal in forming bonds between atoms. They dictate the bonding characteristics within **molecular structures**, influencing both covalent and ionic bonding types. This understanding leads to insights into an element’s **chemical behavior** and reactivity in various conditions, crucial for mastering foundational chemistry concepts.
3. Can the number of valence electrons change in elements?
Regarding elements in stable forms, the total number of **valence electrons** does not change; however, in reactive forms or ionized states, as atoms gain or lose electrons, their effective number can appear altered, leading to differing oxidation states. Understanding this can clarify behavioral changes during **chemical reactions**.
4. What is **Lewis dot notation**, and why is it important?
**Lewis dot notation** is a graphical representation of an element’s **valence electrons** and its bonding arrangement. It provides a useful visual tool for predicting how atoms will bond and interact in forming **molecular configurations**, which is essential for students studying chemical models and ionic vs. covalent interactions.
5. How can I use the octet rule in my studies?
The **octet rule** can help simplify the prediction of atom interactions and stability. By ensuring that atoms either gain, lose, or share electrons to complete their valence shell, students can easily assess potential bonds and reactions of elements in various chemical compounds. Using this approach reinforces understanding within the framework of atomic structure and chemical bonding.
Key Takeaways
- Valence electrons are crucial for understanding chemical bonding and properties.
- Counting techniques, including using the periodic table and **Lewis dot structures**, simplify finding valence electrons.
- Grasping how valence electrons influence **chemical bonding** helps in predicting molecular interactions.
- Applying the octet rule provides a foundational strategy for evaluating stability and reactivity.
- Understanding electronegativity and its relationship with valence electrons is key to comprehending molecular polarity and behavior.
For a deeper dive into the topic, utilize these educational resources. An engaging visual can also be helpful while studying:
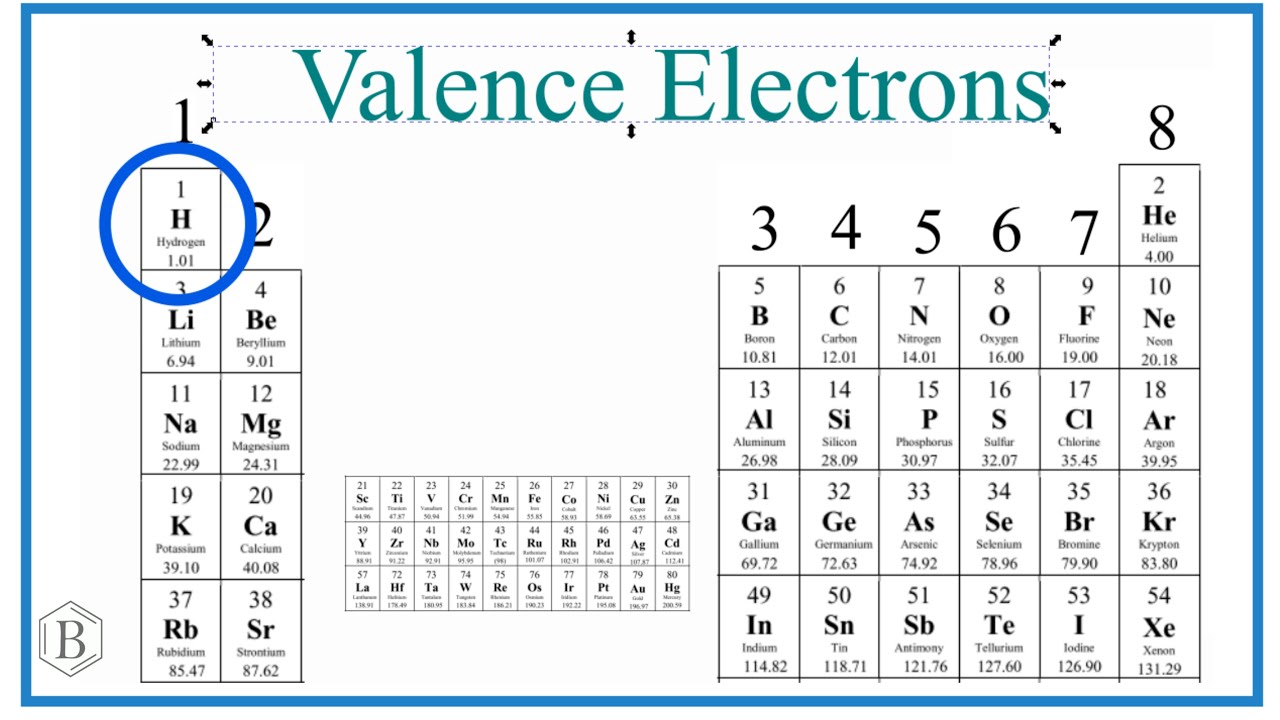
This overview mirrors fundamental and advanced chemistry principles, geared towards students striving to excel in their understanding of **valence electrons** and their essential applications in the field.
To continue exploring the intricacies of chemistry, delve into our related articles at this link and expand your knowledge on elements and bonds.