Effective Ways to Calculate Molar Mass in 2025: Discover Practical Methods!
Understanding Molar Mass and Its Significance
The concept of **molar mass** is a foundational aspect of chemistry, providing essential insights into the relationships between the amount of substance and its properties. Molar mass is defined as the mass of one mole of a substance, typically expressed in grams per mole (g/mol). Understanding how to **calculate molar mass** is crucial for various applications, including stoichiometry and solution concentration calculations. Each element in the **periodic table** has a specific **atomic mass**, which is integral to determining the **molar mass of compounds**.
The Molar Mass Formula
The **molar mass formula** can be summarized as the sum of the atomic masses of all atoms in a molecule. To calculate the molar mass of a compound, you must first identify its molecular or empirical formula. For example, the molar mass of water (H2O) can be calculated by adding the atomic masses of its constituent elements: two times the molar mass of hydrogen (approximately 1.01 g/mol) plus one times the molar mass of oxygen (approximately 16.00 g/mol), yielding a total of about 18.02 g/mol. Understanding **molar mass units** is essential in conducting accurate laboratory experiments, ensuring that chemical calculations adhere to recognized standards.
Tools for Calculating Molar Mass
<pSeveral tools can aid in the **molar mass calculation** process. Online calculators and software for chemistry calculations simplify the task by allowing users to input molecular formulas directly and receive results instantly. Educational resources, including chemistry textbooks and study guides for chemistry, provide further guidance on this subject. In addition, laboratory techniques such as spectroscopy methods can help experimentally determine molar mass using different analytical techniques.
Practical Methods for Calculating Molar Mass
Calculating molar mass can be approached through various practical methods tailored to different types of chemical calculations. By following systematic steps, chemists can efficiently derive the necessary values for their experiments.
Step-by-Step Guide to Finding Molar Mass
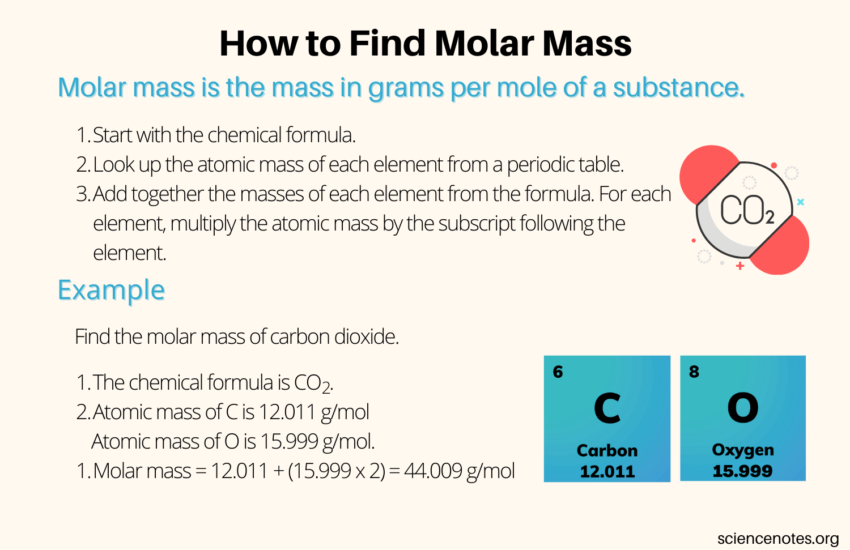
To find molar mass accurately, follow these key steps:
- Identify the **chemical formula** of the substance.
- List the atomic masses of the constituent elements using the **periodic table**.
- Multiply the atomic mass of each element by the number of times it appears in the compound.
- Add together the calculated masses to obtain the total **molar mass** of the compound.
For instance, consider carbon dioxide (CO2). The atomic mass of carbon is approximately 12.01 g/mol, and that of oxygen is about 16.00 g/mol, thus yielding: 12.01 + (2 x 16.00) = 44.01 g/mol for CO2.
Using Molar Mass in Stoichiometry
**Stoichiometry** relies heavily on the concept of molar mass. In reactions, accurate molar mass calculations are vital for determining the **concentration of solutions** and the proportions of reactants and products. For instance, to convert moles to grams, the formula used is: weight (g) = moles x **molar mass**. This relationship aids in finding limiting reactants and calculating reaction yields, making molar mass crucial for successful chemical reactions and ensuring appropriate measurement of substances.
Molar Mass of Elements vs. Compounds
<pUnderstanding the difference between the **molar mass of elements** and that of compounds is key. Elements have a defined molar mass that corresponds to their atomic mass listed on the periodic table, while **molar mass of compounds** involves summing the atomic masses of multiple elements.
Molar Mass Examples for Common Substances
Let’s explore a few common substances to illustrate the differences clearly:
- **Hydrogen (H)**: 1.008 g/mol
- **Oxygen (O)**: 16.00 g/mol
- **Carbon Dioxide (CO2)**: 44.01 g/mol
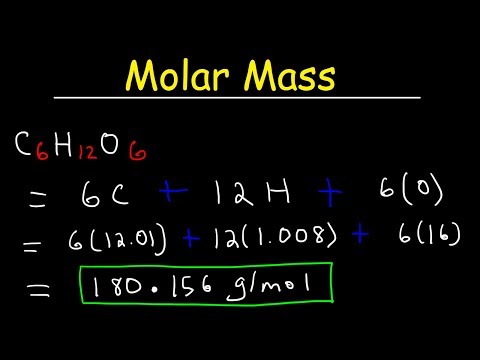
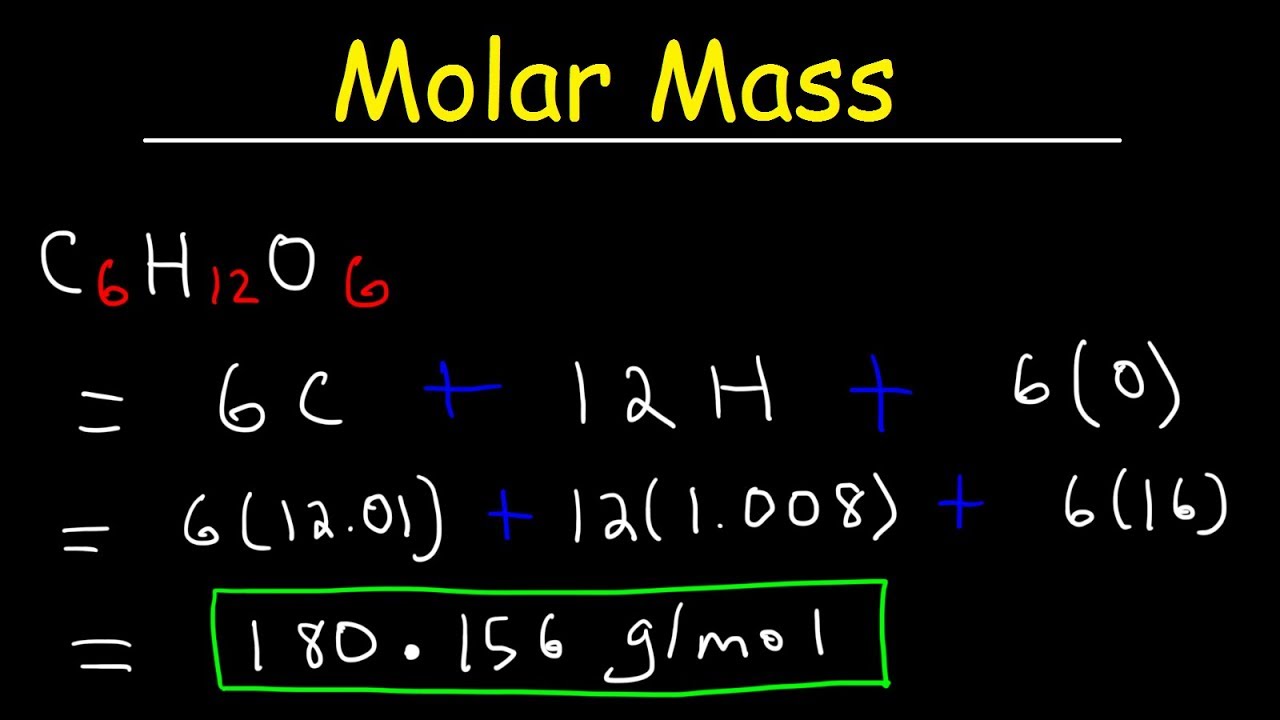
- **Glucose (C6H12O6)**: Approximately 180.18 g/mol
This distinction helps chemists accurately measure, manipulate, and utilize **relative atomic mass** for various experimental and theoretical applications.
Importance of Molar Mass in Real-World Applications
The significance of **molar mass** extends beyond educational contexts; it plays an integral role in research and industry. For example, biotechnology relies on understanding **chemical properties** to synthesize drugs and develop new materials. **Density and molar mass** relationships assist in predicting how substances interact within mixtures. Furthermore, environmental scientists and chemists utilize molar mass in analyzing pollutants, which can help mitigate environmental impacts.
FAQ
1. What is the difference between molar mass and molecular weight?
While often used interchangeably, **molar mass** refers to the mass of one mole of a substance expressed in grams per mole (g/mol), whereas **molecular weight** is a dimensionless quantity that reflects the mass of a molecule relative to the unified atomic mass unit. The distinction becomes relevant in accurate measurements and calculations in advanced chemistry discussions.
2. How do you convert moles to grams using molar mass?
To convert moles to grams, use the formula: grams = moles x **molar mass**. For effective **molar mass calculations**, determine moles from given quantities, then multiply by the molar mass of the substance involved to achieve gram measurements, fundamental in stoichiometric calculations.
3. Why is molar mass important in chemical reactions?
The significance of **molar mass** in reactions stems from its capacity to determine quantitative relationships between reactants and products, facilitating accurate **chemical calculations**. Understanding molar mass allows chemists to balance equations effectively and predict the yield of reactions.
4. Can molar mass be measured experimentally?
Yes, methods such as mass spectrometry, boiling point elevation, and freezing point depression allow for the **experimental determination of molar mass**. These techniques provide insights into the behavior of substances, reinforcing the importance of understanding how to find molar mass for various chemical compositions.
5. What role does Avogadro’s number play in calculating molar mass?
Avogadro’s number, approximately 6.022 x 1023, represents the number of particles in one mole of a substance. It provides a foundation for establishing the relationship between the mass of a substance and the amount in moles. This fundamental concept connects to calculations of the **weight of a mole**, simplifying conversions and applications in **molecular chemistry**.
In summary, mastering how to **calculate molar mass** significantly enhances chemical understanding and application. By adopting these methods, you can ensure accuracy in projects ranging from experimental setups to practical applications in various fields. Consider implementing these techniques in your future endeavors; they are invaluable for students, educators, and professionals alike.